Laboratory controls must be followed and documented at enough time of effectiveness. Any departures from the above mentioned-described processes should be documented and described.
All through the GMP restrictions, a variety of file sorts have specified retention durations. These retention intervals become the minimum needs for file retention.
If this transformation influences the end result of information, an investigation shall be initiated and, post-investigation, correction of your mistake shall be manufactured and the improve shall be countersigned by a supervisor.
An impurity profile describing the identified and unidentified impurities existing in a typical batch produced by a particular managed generation approach must normally be recognized for every API. The impurity profile need to incorporate the identity or some qualitative analytical designation (e.
Any substances related to the operation of equipment, like lubricants, heating fluids or coolants, shouldn't Get in touch with intermediates or APIs In order to alter the caliber of APIs or intermediates outside of the official or other founded technical specs.
Typographical Glitches/Skipped Entries noticed in “authorised” documents through activity, shall be corrected/stuffed in (as relevant) within the respective web site via the involved supervisor, which include signature and date and shall be verified because of the QA Manager/designee.
Examine the significance of ISO criteria during the pharmaceutical market. Learn the way ISO compliance improves quality, protection, and world marketplace accessibility inside our in depth manual.
Use of a stamp to exchange manual relationship, initials or signature on GMP documents, other than in the situation of validated electronic signature.
At the least one particular check to verify the identification of each batch of material really should be conducted, apart from the elements explained below. A provider's certificate of analysis
By adhering to these pointers and preserving a dedication to steady enhancement, enterprises can exhibit their dedication to producing Protected and significant-high quality merchandise, getting a aggressive edge within the Market.
Output website officer and QC Analysts shall file precise benefits received at enough time of carrying out an exercise, without bias or prejudice.
Properly recognized reserve samples of each API batch should be retained for 1 calendar year following the expiry date on the batch assigned with the manufacturer, or for 3 many years soon after distribution with the batch, whichever is extended.
Validation really should prolong to Those people functions established for being critical to the standard more info and purity on the API.
Ensuring that that all generation deviations are documented and evaluated Which significant deviations are investigated and also the conclusions are recorded
 Sydney Simpson Then & Now!
Sydney Simpson Then & Now! Kelly Le Brock Then & Now!
Kelly Le Brock Then & Now!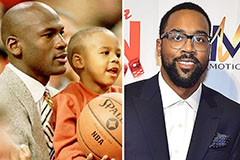 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!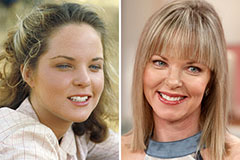 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Catherine Bach Then & Now!
Catherine Bach Then & Now!